Realgar
Mineralogical Data
Chemical Formula:
As4S4
Crystal System:
Monoclinic
Morphology:
Prismatic crystals, grains, crusts,
& earthy masses
Appearance:
Transparent; dark red to orange-red
Moh's Hardness:
1½ - 2
Density:
3.56 g/cm3 (Measured)
3.59 g/cm3 (Calculated)
Tenacity:
Sectile
Other Names:
ruby sulfur
Related Links
Realgar (arsenic II sulfide) is a ruby red sulfide mineral. It is frequently associated with calcite, quartz, and other sulfides - such as stibinite, pyrite, sphalerite, and orpiment. Realgar has been used as a pigment for centuries, despite its inherent photosensitivity.

Bright red translucent crystals of realgar covered by a pararealgar coating. From the Estonian Museum of Natural History, specimen # 201705
Photo-induced Structural Change
Realgar alters to orange pararealgar (As4S4) when exposed to light, regardless of the presence of oxygen (Pratesi and Zoppi 2015). Yet achieving a truely oxygen-free environment is difficult. Even in an ultrahigh vacuum, not all of the oxygen is removed (Kyono et al. 2005). Small concentrations of oxygen can be present on the realgar surface.
When first exposed to light and oxygen, realgar will initially alter to uzonite (As4S5) and arsenolite (As2O3) before producing pararealgar and a sulfur atom (Kyono et al. 2005; Kyono 2007; Jovanovski & Makreski 2020). Light provides the system sufficient energy to break the As-As bonds required for product formation.
5As4S4 (realgar) + 3O2 => 4As4S5 (uzonite) + 2As2O3 (arsenolite)
As4S5 (uzonite) => As4S4 (pararealgar) + S
Deterioraton continues - even when realgar is no longer exposed to light (Jovanovski & Makreski 2020) - via a cyclical, self-sustaining pair of reactions. The sulfur atom inserts itself into a realgar molecule, producing uzonite. Uzonite then soon decays into pararealgar and produces a new sulfur atom.
As4S4 (realgar) + S => As4S5 (uzonite)
As4S5 (uzonite) => As4S4 (pararealgar) + S
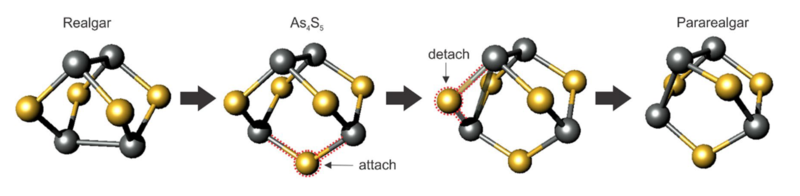
A schematic representation of realgar's alteration to pararealgar through uzonite. The sulfur radical attaches itself to the molecule by breaking an As-As bond. Another sulfur ion is the released, resulting in a new bond between a pair of arsenic ions.
from Jovaniski & Makreski 2020
Reaction Factors
Wavelength
Douglass et al. (1992) found that realgar alteration occurs when the mineral is exposed to wavelengths of 500-670nm. These wavelengths have the properties necessary to hit and break As-As bonds, rather than being reflected or refracted by the crystal.
However, Douglass et al. (1992) also states that:
- they were unable to determine an upper (red-end) wavelength limit for the reaction
- that the reaction is much slower when exposed to wavelengths >610nm (orange-red light)
- it is likely that a certain number of photons are required to cause the reaction.
Intensity
Kyono (2007) looked further into the effects of light intensity on reaction rate. He exposed single realgar crystals to a halogen bulb (150W; 350-850nm) at a distance of 12cm from the sample. Three power densities were reviewed: 5, 10, and 30 W/m2. He found that lower intensity light lengthens the time required for realgar to react.
In the table below, I’ve calculated* what this would equate to in terms of watts and lux to get a better idea of how this would be applied in a museum setting.
|
Watts * |
meters away |
Watt/meter2 |
Lux * |
Hours until disintegration |
|
1.51 |
0.12 |
1 |
36.19 |
5700 |
|
7.54 |
0.12 |
5 |
180.96 |
648 |
|
15.08 |
0.12 |
10 |
361.91 |
276 |
|
45.24 |
0.12 |
30 |
1085.73 |
60 |
|
150 |
0.12 |
99.47 |
30000 |
- |
|
150.8 |
0.12 |
100 |
3619.11 |
30 |
|
1055.58 |
0.12 |
700 |
25333.8 |
7 |
Halogen lamps have a typical luminous efficacy of 16-24lm/W (found here). I used the higher value during calculation as a means of estimating the maximum illumination a sample would receive.
I would like to stress that this is with a halogen bulb. All of the articles I’ve read have only exposed realgar to either sunlight or halogen light bulbs. So while we can calculate what the equivalent lux values are for different types of bulbs, we cannot predict what a specimen lifetime might be under such circumstance. We also cannot predict its lifetime if the specimen is only exposed to certain wavelengths (i.e. through the use of filters), rather than the whole visible spectrum (as is the case with halogen bulbs).
Locality
Specimens of α- and β-realgar from waste dumps at Mina Alacrán, Pampa Larga, Chile were claimed by Alan Clark (1970) to be stable despite years of light exposure. Clark estimated that the specimens were exposed to sunlight for about 20 years in the dumps. He then further exposed them for over two years ex situ. Powder X-ray diffraction patterns determined that the collected realgar specimens (both the α- and β- polymorphs) were of high purity, which was confirmed by XRF analysis.
However, Popova et al. (1986) determined that these samples were in fact a new mineral species, alacránite (As8S9), which has a similar PXRD pattern to β-realgar. In addition, Clark mentions that 5 years earlier, Cahoon observed realgar specimens from Pampa Larga (“probably from Mina Alacrán or the nearby Mina Descubridora”; Clark 1970, pg. 1343) deteriorating after a month’s exposure to sunlight. These in conjunction confirm that Clark’s samples were indeed not realgar.
As of yet, no stable localities have been determined.
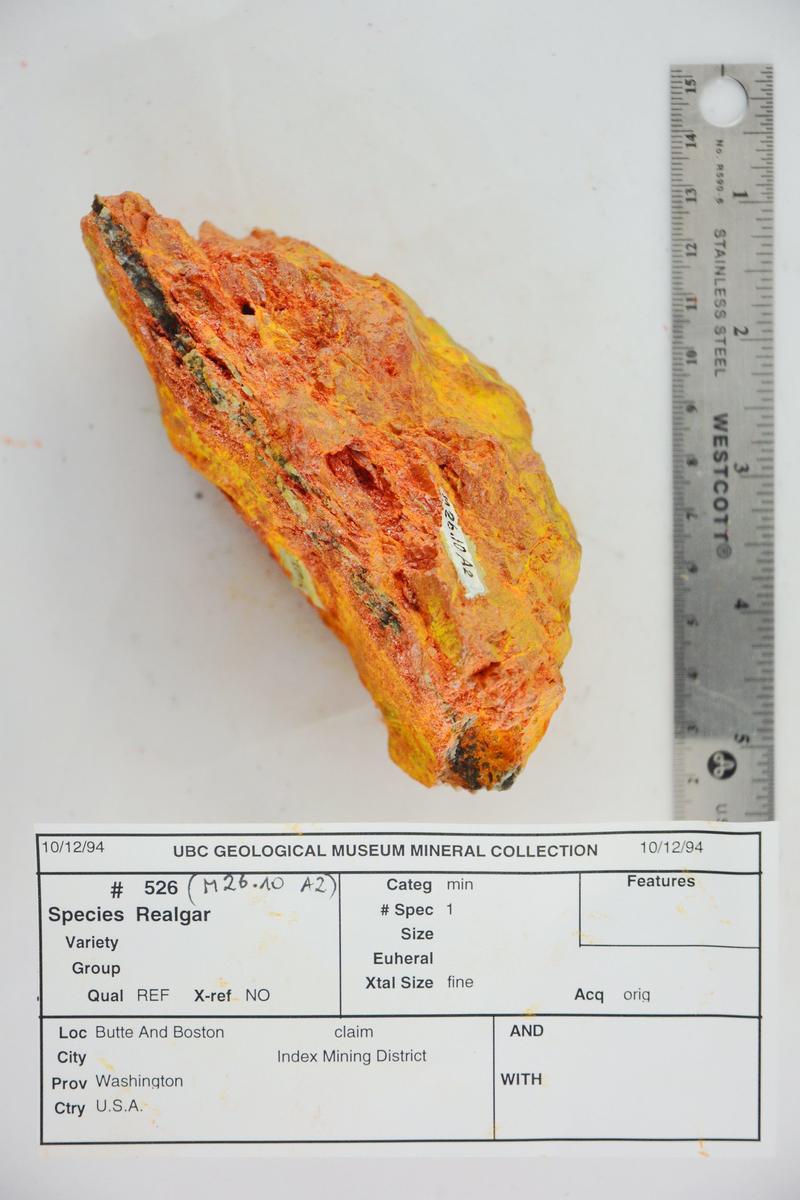
A specimen of realgar significantly altered to pararealgar. From the Pacific Museum of Earth; accession # M26.10 A2
Purity
Regarding purity, modern experiments using 99+% pure realgar have found the mineral to be photosensitive. In addition, inclusions of Sb do not effect light-sensitivity one way or another (Zoppi and Pratesi 2012, Pratesi and Zoppi 2015). It is possible that other inclusions or impurities could induce photostability, but it is unlikely given the current realgar reaction hypothesis (Kyono et al. 2005, Bonazzi et al. 2006).
Recommendations
In summary, there isn't a way to display realgar under illumination which prevents alteration from occurring. But even though reactions still occur in the dark, light exposure significantly increases the deterioration rate. Thus by reducing light exposure and using dim, low intensity light, it should be possible to slow the reaction rate and extend the specimen’s lifetime.
* Equations used:
|
Power Density at a Distance |
|
X = power density P = power r = distance between object and light source |
|
|
E = illuminance P = power n = luminous efficacy A = area being illuminated |
References:
-
Bonazzi, P., Bindi, L., Pratesi, G., and Menchetti, S., 2006. Light-induced changes in molecular arsenic sulfides: State of the art and new evidence by single-crystal X-ray diffraction. American Mineralogist, 91 (8–9), 1323–1330.
-
Clark, A.H., 1970. Alpha-arsenic sulfide, from Mina Alacran, Pampa Larga, Chile. American Mineralogist, 55, 1338–1344.
-
Douglass, D.L., Chichang Shing, and Ge Wang, 1992. The light-induced alteration of realgar to pararealgar. American Mineralogist, 77 (11–12), 1266–1274.
-
Jovanovski, G. and Makreski, P., 2020. Intriguing minerals: photoinduced solid-state transition of realgar to pararealgar—direct atomic scale observation and visualization. ChemTexts, 6 (1).
-
Kyono, A., 2007. Experimental study of the effect of light intensity on arsenic sulfide (As4S4) alteration. Journal of Photochemistry and Photobiology A: Chemistry, 189 (1), 15–22.
-
Kyono, A., Kimata, M., and Hatta, T., 2005. Light-induced degradation dynamics in realgar: In situ structural investigation using single-crystal X-ray diffraction study and X-ray photoelectron spectroscopy. American Mineralogist, 90 (10), 1563–1570.
-
Pratesi, G. and Zoppi, M., 2015. An insight into the inverse transformation of realgar altered by light. American Mineralogist, 100 (5–6), 1222–1229.
-
Zoppi, M. and Pratesi, G., 2012. The dual behavior of the β-As4S4 altered by light. American Mineralogist, 97, 890–896.