Cinnabar

Cinnabar from Ukraine. On public display at the Carnegie Museum of Natural History, Pittsburgh, Pennsylvania, USA

a specimen of massive cinnabar from New Almaden, New Almaden Mining District, Santa Cruz Mountains, Santa Clara County, CA, USA
Mineralogical Data
Chemical Formula:
α-HgS
Crystal System:
Trigonal
Morphology:
Rhombohedral or tabular crystals, granular masses
Appearance:
Transparent to translucent; carmine red
Moh's Hardness:
2-2.5
Density:
8.179 g/cm3 (Measured)
8.20 g/cm3 (Calculated)
Tenacity:
Sectile
Other Names:
Vermilion (when used as a pigment)
Related Links
Cinnabar (mercury II sulfide) is a vibrant red sulfide mineral, long used as the pigment vermillion in paintings and frescoes (Gettens et al. 1972, Saunders and Kirby 2004, Keune and Boon 2005, Radepont et al. 2011, Neiman et al. 2015). However, it is notoriously photosensitive, irreversibly blackening with light exposure.

"(a) "The Adoration of the Magi" by Peter Paul Rubens, Royal Museum of Fine Arts, Antwerp, Belgium; (b) optical photograph of a degraded area near the right sleeve of the figure on the left; (c) higher magnification photograph showing darkened areas and white precipitates on the top surface; (d) optical micrograph of one analyzed sample"
from Radepont et al. 2011
Photosensitivity
Mineral photosensitivity can be caused by the mineral’s composition or structure (as we’ve seen previously with realgar). Photosensitivity can also be attributed to inclusions or impurities. The degree of photosensitivity can also vary with the specimen’s origin (Nassau 1992), formation conditions, and associated mineralogy.
This is the case with cinnabar. Specimens from certain localities have been shown to be photosensitive (Dreyer 1939, McCormack 2000). At these locations, cinnabar is associated with a number of mercury halide minerals (McCormack 2000, Neiman et al. 2015):
- calomel - ([Hg1+]2)Cl2
- comancheite - Hg2+55N3–24(NH2,OH)4(Cl,Br)34
- corderoite - Hg2+3S2Cl2
- eglestonite - ([Hg1+]2)3OCl3(OH)
- kenhsuite - Hg2+3S2Cl2
- kleinite - (Hg2N)(Cl,SO4) · nH2O
- mosesite - (Hg2N)(Cl,SO4,MoO4) · H2O
- radtkeite - Hg2+3S2ICl
- terlinguaite - [Hg3]4+Hg2+Cl2O2
Cinnabar associated with these minerals often contain trace amounts (~1 wt%) of alkali halogens, usually chlorides (Dreyer 1939, McCormack 2000, Keune and Boon 2005).
At these concentrations, halogens are integral catalysts for the photochemical redox reaction which produces the darkening (Keune and Boon 2005, Anaf et al. 2013, Neiman et al. 2015). Moisture also plays a key role in the reaction (Saunders and Kirby 2004, Neiman et al. 2015); increasing RH results in a greater colour change from red to black.
The surficial blackening of cinnabar is attributed to the formation of colloidal metallic mercury nanoparticles (Dreyer 1939, Keune and Boon 2005, Anaf et al. 2013, Da Pieve et al. 2013, Neiman et al. 2015), rather than the long assumed metacinnabar—βHgS— (Gettens et al. 1972), calomel, or corderite (Da Pieve et al. 2013, Neiman et al. 2015); although the latter two are common reaction products.
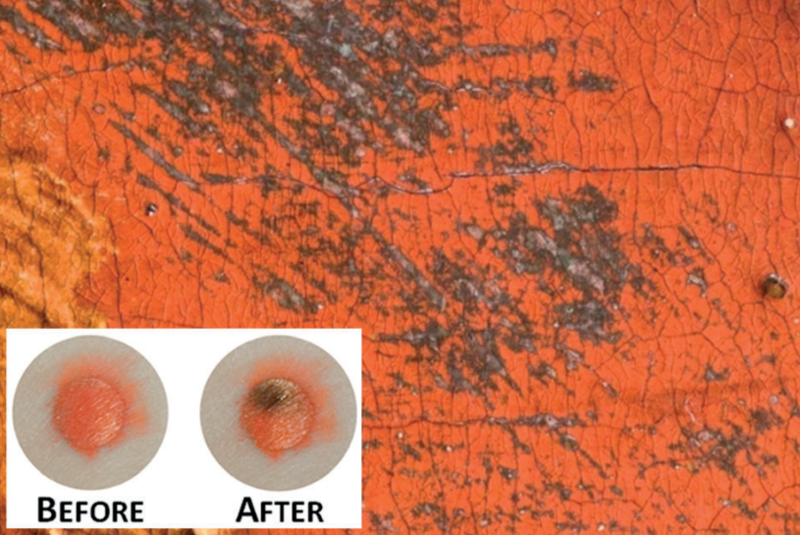
An example of cinnabar’s alteration from red to black on Ruben’s painting ‘The Adoration of the Magi’. The inset shows Anaf’s experimental samples of cinnabar before and after light and chloride exposure. In both instances, the black reaction product was identified as metallic mercury.
from Anaf et al. 2013
The primary reaction sequence is as follows:
First, cinnabar transforms into corderoite (and or kenhsuite) upon exposure to light, moisture, and chloride ions (Keune and Boon 2005, Radepont et al. 2011).
However, corderoite is structurally unstable when exposed to light and oxygen (Da Pieve et al. 2013), and will degrade into calomel, metallic mercury, and sulfur (Keune and Boon 2005).
Kenhsuite, an even less stable polymorph of corderoite (Radepont et al. 2011), is also photosensitive and will likewise decompose; first into corderoite and then into the subsequent products.
Calomel can then be further reduced to mercuric chloride and metallic mercury:
Additional reactions can occur alongside these and produce a number of other chlorine- and sulfur-containing species (Keune and Boon 2005, Anaf et al. 2013), which may or may not contain mercury (Radepont et al. 2011).
Inhibition
The reaction is dependent on the presence of light, moisture, and halogens to occur. If one element is missing, the reaction will not occur. This can be advantageous in designing a preservation strategy, yet is more difficult that it first seems.
If a photosensitive specimen is in storage, the easiest solution would be to store it in a light-proof container. But if a specimen were to go on display, light cannot be removed.
Moisture could be removed from the environment, however the minimum moisture requirement has yet to be determined. Even at 35%RH, cinnabar will deteriorate in a matter of hours if exposed to light and chloride (Neiman et al. 2015).
Halogens are just as difficult to remove, as they can come from various sources: associated minerals and materials, inclusions and impurities, the air, etc. And only a very small quantity of halogens is needed to initiate the reaction. While again the exact lower threshold has not been determined, McCormack (2000) showed that specimens containing less than 0.01 wt% (± 0.01) were light stable, whereas those containing ≥0.04 wt% (± 0.01) were photosensitive.
Recommendations
As such, only pure, non-photosensitive cinnabar specimens should be displayed.
Any cinnabar testing positive for >0.05 wt% Cl should be considered for light-proof storage, especially those where the chloride content is >1 wt%.
If black metallic mercury is already present on the specimen’s surface, it is best to not remove it. The reaction is surficial; only affecting the cinnabar molecules that have been exposed to light. Below the layer of metallic mercury is still unreacted cinnabar. Exposing a fresh cinnabar surface to light will only result in further reaction and specimen loss.
References:
- Anaf, W., Janssens, K., and De Wael, K., 2013. Formation of metallic mercury during photodegradation/photodarkening of α-HgS: Electrochemical evidence. Angewandte Chemie - International Edition, 52 (48), 12568–12571.
- Dreyer, R.M., 1939. Darkening of cinnabar in sunlight. American Mineralogist, 24, 457–460.
- Gettens, R.J., Feller, R.L., and Chase, W.T., 1972. Vermilion and cinnabar. Studies in Conservation, 17, 45–69.
- Keune, K. and Boon, J.J., 2005. Analytical imaging studies clarifying the process of the darkening of vermilion in paintings. Analytical Chemistry, 77 (15), 4742–4750.
- McCormack, J.K., 2000. The darkening of cinnabar in sunlight. Mineralium Deposita, 35 (8), 796–798.
- Nassau, K., 1992. Conserving light sensitive minerals and gems. In: F.M.. Howie, ed. The Care and Conservation of Geological Materials: Minerals, Rocks, Meteorites and Lunar Finds. Oxford: Butterworth-Heinemann, 11–24.
- Neiman, M.K., Balonis, M., and Kakoulli, I., 2015. Cinnabar alteration in archaeological wall paintings: an experimental and theoretical approach. Applied Physics A: Materials Science and Processing, 121 (3), 915–938.
- Da Pieve, F., Hogan, C., Lamoen, D., Verbeeck, J., Vanmeert, F., Radepont, M., Cotte, M., Janssens, K., Gonze, X., and Van Tendeloo, G., 2013. Casting light on the darkening of colors in historical paintings. Physical Review Letters, 111 (20).
- Radepont, M., De Nolf, W., Janssens, K., Van Der Snickt, G., Coquinot, Y., Klaassen, L., and Cotte, M., 2011. The use of microscopic X-ray diffraction for the study of HgS and its degradation products corderoite (α-Hg3S2Cl2), kenhsuite (γ-Hg3S2Cl2) and calomel (Hg2Cl2) in historical paintings. Journal of Analytical Atomic Spectrometry, 26 (5), 959–968.
- Saunders, D. and Kirby, J., 2004. The Effect of Relative Humidty on Artists’ Pigments. In: J. Green and D. Davies, eds. National Gallery Technical Bulletin. London: National Gallery Company Ltd, 62–72.








