Chalcanthite

Mineralogical Data
Chemical Formula:
CuSO4 · 5H2O
Crystal System:
Triclinc
Morphology:
Prismatic crystals, stalactitic, veins, or granular masses
Appearance:
Translucent; pale-dark blue
Moh's Hardness:
2.5
Density:
2.286 g/cm3 (Measured)
2.282 g/cm3 (Calculated)
Tenacity:
brittle
Other Names:
blue vitriol
copper vitriol
Related Links
Chalcanthite (copper II sulfate pentahydrate) is a bright blue hydrated sulfate mineral, often found in mines and near-surface environments. In these conditions, chalcanthite is often present as an alteration product of sulfide minerals such as pyrite (FeS2) and chalcopyrite (CuFeS2) (Blount 1993; Jambor et al. 2000; Chou et al. 2013). But it is often only an intermediary product and will react further in the presence of moisture, oxygen, sulfuric acid, and or other mineral species (Jambor et al. 2000; Chou et al. 2013).
Dehydration
In a museum environment, chalcanthite is usually only at risk of dehydrating into lower hydrates, such as bonattite (CuSO4·3H2O). However, dehydration does not occur until below 30%RH at 20°C (or 35%RH at 30°C; Chou et al. 2002).
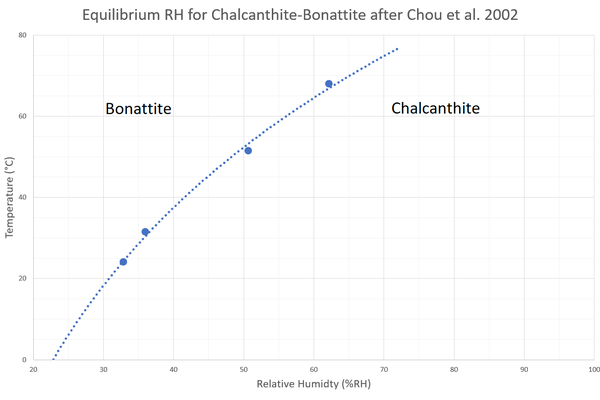
Last summer I performed a dehydration experiment of chalcanthite at 35%RH & 30°C for my MRes dissertation. Surprisingly, I was unable to produce any signs of deterioration! This means that there must be a degree of ‘undercooling’ required to produce sufficient energy to induce detectable dehydration.
Undercooling is when a material requires environmental conditions (such as temperature, moisture, pressure, or pH) just past the material’s stability boundary to react or change state. While there is theoretically enough energy at boundary conditions for a reaction to occur, it may not be enough to initiate it. Or the reaction is occurring, but just at a very slow rate.
In short, average room conditions (room temperature & 50%RH) will preserve 100% pure chalcanthite, and mild variations in RH should not produce any chemical changes.
The Effect of Impurites
If a large quantity of other metal ions is present (especially iron), the specimen may be unstable under average room conditions. While I have not yet found any references mentioning the exact copper to metal ion ratio to produce instability, Eckel (1933) shows that cuprian melanterite (pisanite)—where Fe:Cu is 18:7—can dehydrate at 45%RH & 22°C. This aligns with anecdotal observations of chalcanthite specimens in collection stores; some have dehydrated while others have not.


These two chalcanthite specimens are from National Museum Wales Cardiff.
The first one has remained in good condition while the second one has dehydrated to a pale green-blue. X-ray diffraction analysis was performed on a sample of the later. It was determined that that the specimen contains a large quantity of iron in additon to copper. The hydrated iron sulfate, melanterite (FeSO4 · 7H2O), has dehydrated into siderotil (FeSO4 · 5H2O).
Recommendations
I recommend storing chalcanthite specimens at room temperature & 45-60%RH. I also would suggest a form of elemental analysis to determine the purity of the specimen. If iron is detected, I’d caution towards 60%RH. The higher the iron content, the higher the RH should be.
But if analysis is not possible, frequent monitoring for signs of dehydration could be implemented instead. The rate of dehydration will depend on the ambient storage conditions. The drier the air, the faster the reaction. As such, chalcanthite can dehydrate in a matter of days or over a number of months. If the specimen has been determined to be stable after this period, regular institutional monitoring routines can be used.
References:
- Blount, A.M., 1993. Nature of the alterations which form on pyrite and marcasite during collection storage. Collection Forum, 9 (1), 1–16.
- Chou, I.M., Seal, I.R., & Hemingway, B.S., 2002. Determination of melanterite-rozenite and chalcanthite-bonattite equilibria by humidity measurements at 0.1 MPa. American Mineralogist, 87 (1), 108–114.
- Chou, I.M., Seal, R.R., & Wang, A., 2013. The stability of sulfate and hydrated sulfate minerals near ambient conditions and their significance in environmental and planetary sciences. Journal of Asian Earth Sciences, 62, 734–758.
- Eckel, E.B., 1933. Stability relations of a Colorado pisanite (cuprian melanterite). American Mineralogist, 18 (10), 449–454.
- Jambor, J.L., Nordstrom, D.K., & Alpers, C.N., 2000. Metal-sulfate Salts from Sulfide Mineral Oxidation. In: C.N. Alpers, J.L. Jambor, & D.K. Nordstrom, eds. Sulfate Minerals: Crystallography, Geochemistry, and Envrionmental Significance. Washington D.C.: Mineralogical Society of America, 303–350.







